About us
Our philosophy
Since the creation of our company in Barcelona in 1982, we have been at the forefront of the design and development of acupuncture and physiotherapy equipment. Our approach focuses on listening and responding to the needs of health professionals, adapting quickly to new trends and technical requirements. In collaboration with universities and professional bodies, we continue to innovate and refine our tools to ensure maximum efficiency and comfort.
Quality
At Agupunt, quality is not just a standard; it is our signature. We are ISO 13485 and MDSAP certified. In addition, our products comply with the Medical Devices Regulation 2017/745. All our needles are approved by the FDA and Health Canada, among other international organisations.
RD
Our track record as a leading needle manufacturer constantly drives us towards innovation, resulting in a diversified range of needles meticulously adapted to various specialised techniques.
Continuous progress is at the heart of our commitment, responding with agility to the emerging demands of professionals in fields such as physiotherapy, acupuncture, podiatry, veterinary medicine and neurology, among others.
Thanks to our own production facilities and a dedicated RD team, we have the flexibility and resources to research and introduce new product lines.
1982: Launch of the Agupunt brand as an ASP needle manufacturer.
1986: We started importing and distributing other products, in order to offer a complete range of acupuncture and physiotherapy treatments.
1995: Offices and warehouse set up in Shanghai
2000: AGUPUNT’s first website of the company’s current CEO is created and published.
2006: 25 years of experience‘ The pioneers in Spain’.
2008: APS needle innovation projects the dry start
2010: Offices and shop established A CC áspid mi Barcelona
2012: Design and manufacture of machines and needles for PPE and strategy for international expansion (6 countries).
2014: Launch of ‘regular’ dry needling’.
2015: Launch of ‘shallow’ Dry Needling needles and entered the US market
2016: Launch of dry needling needles ‘Safety tube’.
2018: Launch of the invasive electrotherapy device ‘APS e4’.
2019: Launch of Click Dry Needling needles and opening of offices in Florida
2020: Launch of dry needle puncture for superficial fascia
2021: Agupunt obtains MDSAP certification to market the product in Canada and the United States – United Kingdom
2022: New logistics facilities in Tarragona in 2022. Start of marketing in Switzerland
Our logo has evolved over the 40 years of our history, always trying to convey the core business of the business, dry needling.
With this latest development, we want to continue to highlight the dot, or even integrate it into the brand, as a symbol of precision for the expansion of energy in care, thus making it a symbol and an iconic element.
This leads us to make our product a service that functions as a guarantee of quality and contributes to strengthening the relationship between the brand and the professional, not only as a guarantee of the product but also of the processing.
It symbolises the puncture point and the energetic expansion that occurs during treatment.
Reinforcing our main differentiator, the sharpness, which provides precision and safety to the specialist.
Our experience of more than 40 years in the manufacture of needles has enabled us to innovate and develop a wide range of needles adapted to each technique.
As a manufacturer, we control all aspects of needle manufacture, from design to final service, so that we can guarantee the quality of every needle we produce.
We offer a high quality product, created and designed in highly resistant, more durable and more flexible surgical steel. An alloy specially created for our needles, finished with a specific sharpening. The result is an engineered needle that does no damage. If a needle is subjected to micro – breaks during the puncture and the skin suffers.
We manufacture with 304H stainless steel to ensure safe and efficient operation for use in different techniques.
Surface treatment on the entire needle body, suitable to meet the requirements of each technique.
The shape of the needle tip is specific to each technique, offering the user a specific tool.
Different handles for different uses. Different shapes, thicknesses, materials and lengths.
Washing the ultrasonic needles after production to remove all unwanted substances.
Ethylene oxide (EO) sterilised needles to ensure safe treatment.
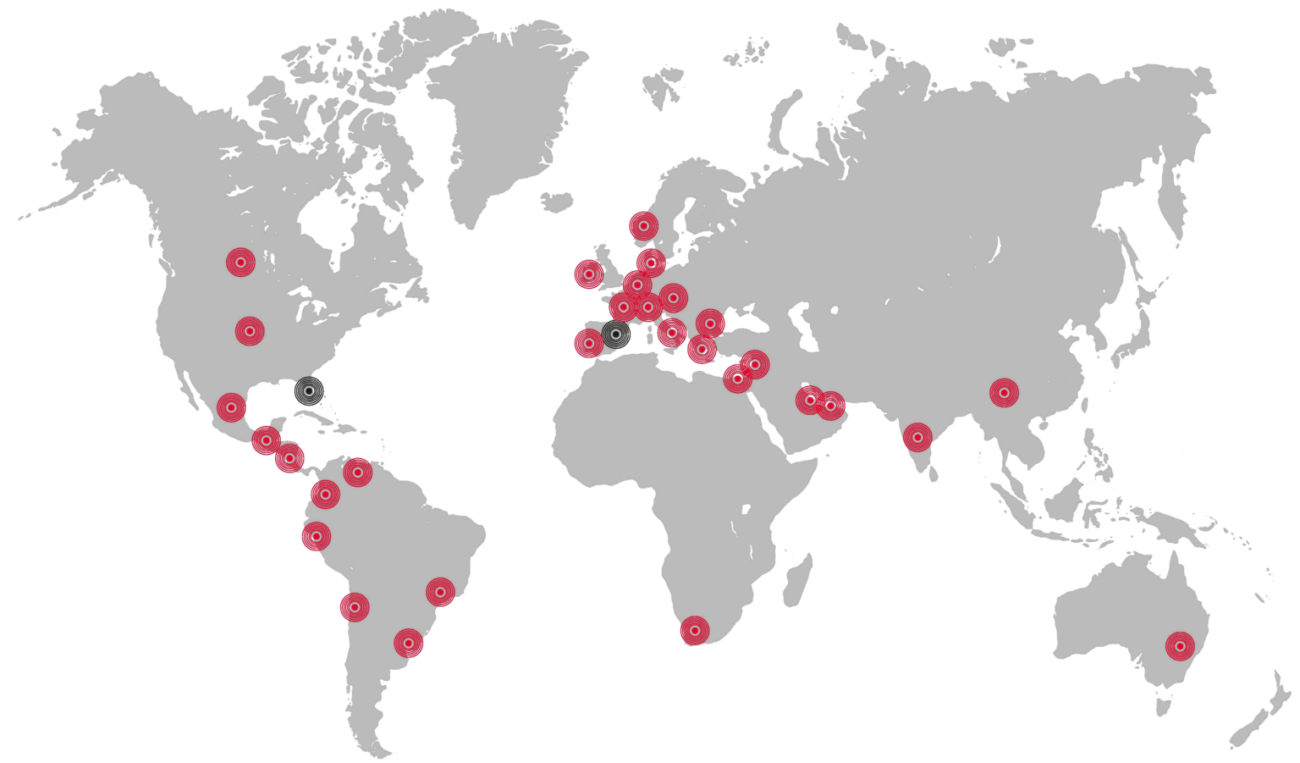
Where we are
Andorra-Barain-Belgium-Brazil-Chile-Colombia-Costa Rica-Ecuador-France-Guatemala-Hungary-Ireland-Italy-Jordan-Kuwait-Latvia-Netherlands-Norway-Peru-Portugal-Poland-Qatar-Saudi Arabia-Switzerland-Turkey-Uruguay-U.S.A.-Venezuela